

Affiliations
ABSTRACT
Although aortoenteric fistula has been recognised for more than 50 years and has been rapidly diagnosed and treated, mortality is still very high due to some serious complications such as sepsis or massive haemorrhage. It has been traditionally classified into two groups; primary (PAEF) and secondary (SAEF). An even rarer subgroup is secondary aortoesophageal fistula (SAEsF), and it presents three characteristic findings which are known as Chiari’s triad. In this case report, we describe a case of SAEsF, which had an atypical presentation and fatal outcome.
Key Words: Abdominal aortic aneurysm, Aortoenteric fistula, Aortoesophageal fistula.
INTRODUCTION
Aortoenteric fistulas (AEFs) can originate from either primary or secondary causes. Primary AEF (PAEF) occurs spontaneously through a combination of direct frictional mechanical forces and aortic inflammation.1 Secondary AEFs (SAEFs) are more common and in this type, there needs to be a previous endovascular aortic repair. The leading cause is either a direct injury to the bowel caused by graft material or a rupture of enlarging aneurysm into the bowel.2 SAEF is generally not considered in the preliminary diagnosis of patients with upper gastrointestinal bleeding, especially when presenting with some unexpected symptoms.
CASE REPORT
A 74-year man, known to have an aneurysm at the level of thoraco-abdominal aortic junction, presented to the Emergency Department with the chief complaints of having vomited bright-red blood for the last 2-3 days during cough. He also complained of difficulty in swallowing solid food for the last year. He had a history of a stent graft deployment in 2019 due to an aneurysm in the aorta which was first detected in 2010.
In the physical examination, melena was detected on digital rectal examination. No other remarkable finding was noted. His laboratory testing revealed a haemoglobin level of 10.7 g/dl, platelet count of 208,000/ ml, and INR level of 1.22.
Previous tests performed 10 days ago, in order to investigate his dysphagia, showed a haemoglobin level of 13.6 g/dl, which had proved an apparent drop. Compared to the previous imaging, the current contrast-enhanced thoraco-abdominal CT angiography reported an enlargement in the aneurysm and an endoleak (Figure 1). An upper gastrointestinal (Gİ) endoscopy performed on the following day revealed a large mucosal defect (Figure 2). An esophageal self-expandable metallic stent (SEMS) was applied to the defect in order to relieve obstruction and multiple biopsies were taken. The level of mucosal defect exactly matched to the level of stent graft and the patient was diagnosed as secondary aortoesophageal fistula (SAEsF). Pathological evaluation revealed necrosis and did not show any sign of malignancy.
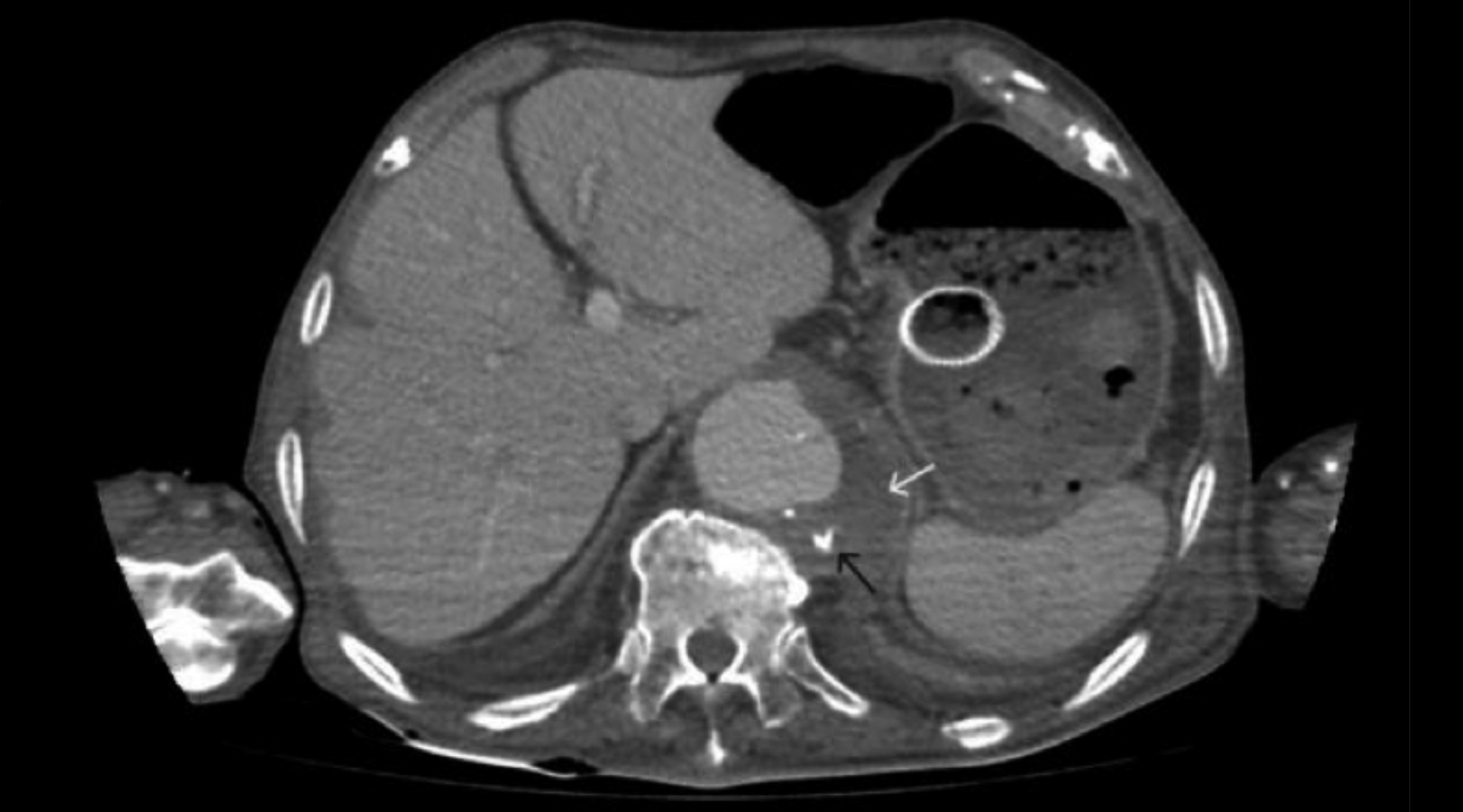 Figure 1: Thoraco-abdominal CT angiography showing enlargement in the hypodensity surrounding the graft (white arrow) and endoleak (black arrow).
Figure 1: Thoraco-abdominal CT angiography showing enlargement in the hypodensity surrounding the graft (white arrow) and endoleak (black arrow).
As the above diagnosis was made, the patient was swiftly transferred to cardiovascular surgery. Ten days later, a control endoscopy was performed, and SEMS was found to be functioning well. Cardiovascular surgeons planned to make a repairing intervention (aortic stent extension); however, during the follow-up, the patient had two massive rebleeding episodes, and he became hemodynamically unstable. The need for the use of heparin in the endovascular repair also caused delay for an intervention, since it would worsen bleeding. After second massive rebleeding, the patient developed haemorrhagic shock and died.
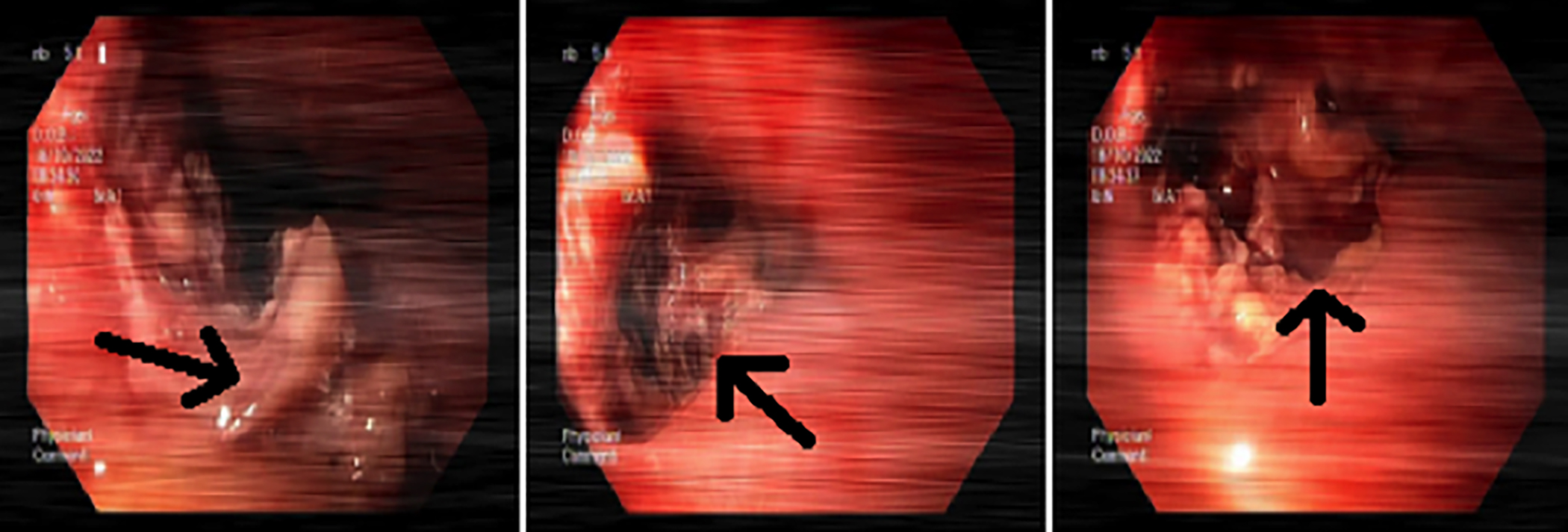 Figure 2: The gastroscopy showing a large mucosal defect (black arrow) at the lower third of esophagus, 31 cm from the incisors.
Figure 2: The gastroscopy showing a large mucosal defect (black arrow) at the lower third of esophagus, 31 cm from the incisors.
DISCUSSION
Endovascular aortic repair was first performed almost three decades ago and had become the basic approach for aortic aneurysms. Early survival results with the endovascular aortic repair were impressive; however, it also predisposes to some serious complications.3
AEFs are not seen commonly and since endovascular surgery improved a lot, SAEFs have overtaken PAEFs in incidence. Among those patients who undergo interventional treatment for their aortic disease, the incidence of SAEFs has been reported to be 0.36% to 1.6%.2 In a systematic literature review, Bergqvist et al. revealed 14 cases of aortoenteric fistulation after aortic stent grafting or stent placement. According to that review, in 6 cases, the mechanism of fistulation was endoleak/aneurysm expansion/rupture; in 5 cases, it was stent-related (defect or fracture of stent); in 2 cases, it was infection/inflammation and for the last case, the cause was not found.1
The incidence of AEsF is much lower than that of AEF, probably because the abdominal aortic pathologies took much more attention of physicians and the number of reconstructive procedures performed for them are more common compared to their thoracic counterparts. In a literature review, Pipinos et al. identified 15 cases of SAEsF between 1976 and 1997, a number significantly smaller than those of the other types of aortopeptic communications.4
Patients with SAEsFs typically present with epigastric pain, sentinel arterial haemorrhage and exsanguination after a symptom-free interval implying massive bleeding (Chiari's triad).5 This patient did not complain of epigastric pain instead, he suffered from dysphagia and cough-induced bright-red blood vomiting.
In conclusion, SAEsF must be remembered by physicians in the differential diagnosis of upper GI bleeding, particularly in patients who have a history of endovascular aortic repair. It is a rare entity and can present with unexpected symptoms.
PATIENT’S CONSENT
Informed consent was obtained from the patient in this case report.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
HHZ: Analysis and interpretation of data, drafting and revising the data.
BO: Analysis and interpretation of data.
KK: Final approval of the work.
All the authors have approved the final version of the manuscript to be published.
REFERENCES