

Affiliations
ABSTRACT
Phyllodes tumour (PT) is a biphasic fibroepithelial tumour of the breast that accounts for 0.3-1% of all primary breast neoplasms. It is subdivided into benign, borderline, and malignant categories based on histologic features including stromal overgrowth, stromal hypercellularity, mitotic count, degree of atypia, and type of margins. Malignant PT (MPT) is characterised by infiltrative margins, marked stromal overgrowth, increased stromal hypercellularity, greater than 10 mitoses/10 high-power fields (HPFs), and moderate to severe atypia. It shows aggressive behaviour with a high risk of local recurrence and metastasis. Surgery with adequate margins is the recommended treatment. Heterologous differentiation is not an uncommon phenomenon in MPT. In addition to classic phyllodes histology, the tumour shows areas of benign/malignant mesenchymal differentiation. MPT containing multinucleated osteoclast-type giant cells (OGCs) is extremely rare. We herein report cases of MPT with OGCs in two young females who had a history of swelling in unilateral breast for two-three years. Histologic examination revealed classic phyllodes areas admixed with a sarcomatous stromal component with OGCs.
Key Words: Malignant phyllodes tumour, Breast, Osteoclast-type giant cells.
INTRODUCTION
Phyllodes Tumour (PT) is a fibroepithelial breast tumour that shows a prominent intracanalicular architectural pattern with leaf-like stromal fronds, capped by luminal epithelial, and outer myoepithelial cells. There is presence of stromal overgrowth and hypercellularity. It accounts for 0.3-1% of all primary breast neoplasms and 2.5% of fibroepithelial neoplasms of the breast.1 PT is divided into benign, borderline and malignant categories based on defined histologic parameters which include the amount of stromal overgrowth, stromal cellularity, mitotic count, degree of atypia, and type of margins.2
MPT shows marked stromal nuclear pleomorphism, marked stromal overgrowth, moderate to severe hypercellularity, increased mitoses (more than 10 mitoses/10 HPFs or 0.5 mm2) and infiltrative borders. MPT is also diagnosed when malignant heterologous elements are present even in the absence of the above-described features.3
These components include sarcoma like rhabdomyosarcoma, osteosarcoma, chondrosarcoma etc. MPT may show multinucleated giant cells that may be bizarre tumour giant cells or rarely, osteoclast-type giant cells (OGCs).4
This case series describes the presence of OGCs in MPT, which is very rare.5
Diagnosis of MPT can be very challenging when multinucleated cells are seen in a breast tumour. The differential diagnoses in such cases include pleomorphic carcinoma of the breast, metaplastic carcinoma of the breast, carcinoma of the breast with choriocarcinomatous features, invasive ductal carcinoma of the breast with OGCs, undifferentiated pleomorphic sarcoma and malignant giant cell tumour of breast/metastatic origin.6
Immunohistochemical stains including Cytokeratin AE1/AE3, Cytokeratin 5/6, p63, Anti-smooth muscle actin (ASMA), Desmin, CD34, S100, HCG, SATB-2 and the presence of classic phyllodes areas help in determining the correct diagnosis.7
This study aims to describe the clinicopathologic and microscopic features and discuss differential diagnoses of this rare entity as misdiagnosis has prognostic implications and differences in clinical management.
CASE 1
A 39-year female presented with a history of cystic swelling in her left breast for 3 years. Wide local excision was done, and the specimen was sent for histopathologic study. It was a skin-covered breast tissue measuring 15 × 12 × 5 cm. Overlying skin was unremarkable. Cut surface revealed a solid cum cystic hemorrhagic lesion that measured 13 × 10 × 4 cm. Deep margin was 0.3 cm and the closest peripheral margin was 0.1 cm away. Microscopic examination revealed a neoplasm with compressed breast ducts lined by epithelium and myoepithelium. The stroma was moderately cellular and comprised of spindle-shaped cells with an abrupt transition to high-grade sarcoma composed of fascicles and sheets of spindle-to-plump cells (Figure 1A). The cells had vesicular nuclei, prominent nucleoli, and moderate eosinophilic cytoplasm. Uniform distribution of OGCs was seen among these atypical cells (Figure 1B). Up to 20 to 22 mitoses were present per 10 HPFs with focal necrosis. Epithelial markers including Pan-cytokeratin, Cytokeratin CAM 5.2, EMA, CK5/6, BerEP4, and p63 were negative in the spindle and giant cells excluding pleomorphic and metaplastic carcinoma and invasive ductal carcinoma of the breast with OGCs. Immunostain HCG was also negative, excluding carcinoma of the breast with choriocarcinomatous features. The giant cells showed CD68 positivity. The tumour cells were negative for S100 stain excluding melanotic and neural differentiation. ASMA and Desmin were also negative excluding smooth and skeletal muscle differentiation. The striking diffuse nuclear positivity of immunostain SATB2 confirmed osteoblastic differentiation (Figure 1C). Patient was contacted to obtain the history of any previous bone swelling or biopsy to exclude the possibility of metastatic malignant giant cell tumour, but there was no such history. Undifferentiated pleomorphic sarcoma was excluded when characteristic phyllodes areas were appreciated in two blocks. Hence, the tumour was best diagnosed as MPT with OGCs. Follow-up was taken after two years. Radical mastectomy with axillary lymph node dissection was done after 2 months of lumpectomy but the patient developed liver metastasis. This was followed by chemotherapy for metastatic disease, however, the patient did not respond well and died in a few months.
CASE 2
A 34-year female presented with a nodular swelling in her right breast for two years. The patient underwent lumpectomy and the specimen was sent for histopathologic evaluation. Grossly, the lump was multinodular, firm and measured 14 × 14 × 11 cm. Cut surface showed a relatively circumscribed tumour with pushing and infiltrative edges, measuring 14 × 13 × 10 cm. The cut surface was tan white with areas of haemorrhage and cystic spaces filled with mucoid material. The tumour was 0.1 cm away from the closest peripheral resection margin. Microscopic examination showed a biphasic fibroepithelial lesion with pushing and infiltrating margins. It showed elongated slit-like compressed breast ducts lined by epithelial and myoepithelial layers, surrounded by moderate to densely cellular stroma. The stroma showed stromal overgrowth with moderate cytological atypia and focal multinucleated giant cells having moderately pleomorphic nuclei with dense eosinophilic cytoplasm. Areas of necrosis and increased mitotic activity of 12/10 HPFs were noted. The case was diagnosed as MPT with a clear margin of 0.1 cm.
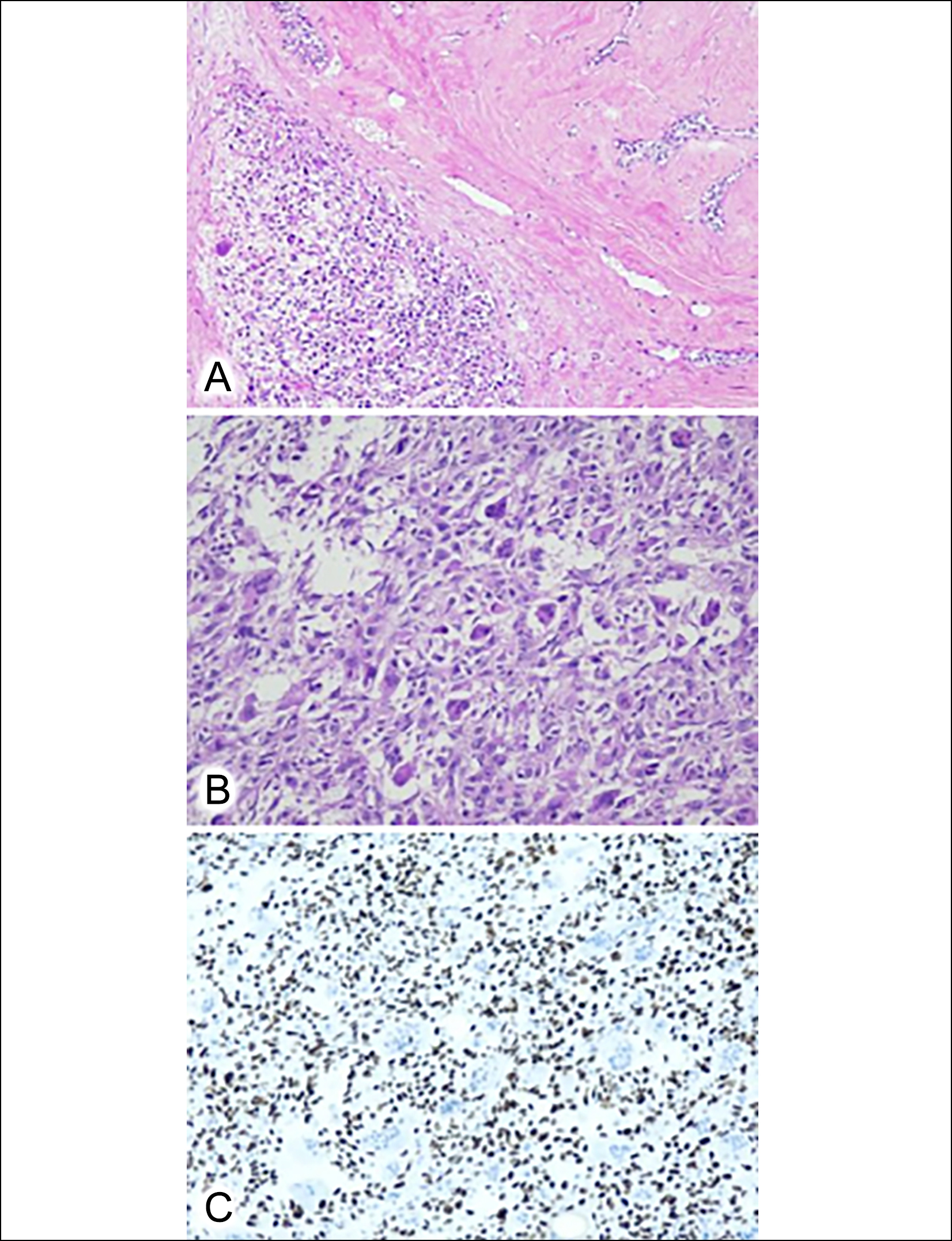 Figure 1: (A) Malignant phyllodes tumour. On the right, Classic phyllodes areas with compressed breast ducts and sclerotic stroma are present with an abrupt transition to sarcoma (H&E, ×10). (B) Stroma containing numerous osteoclast-type giant cells (H&E, ×20). (C) Immunohistochemical stain SATB2 is nuclear positive in stromal cells (Immunostain SATB2, ×20).
Figure 1: (A) Malignant phyllodes tumour. On the right, Classic phyllodes areas with compressed breast ducts and sclerotic stroma are present with an abrupt transition to sarcoma (H&E, ×10). (B) Stroma containing numerous osteoclast-type giant cells (H&E, ×20). (C) Immunohistochemical stain SATB2 is nuclear positive in stromal cells (Immunostain SATB2, ×20).
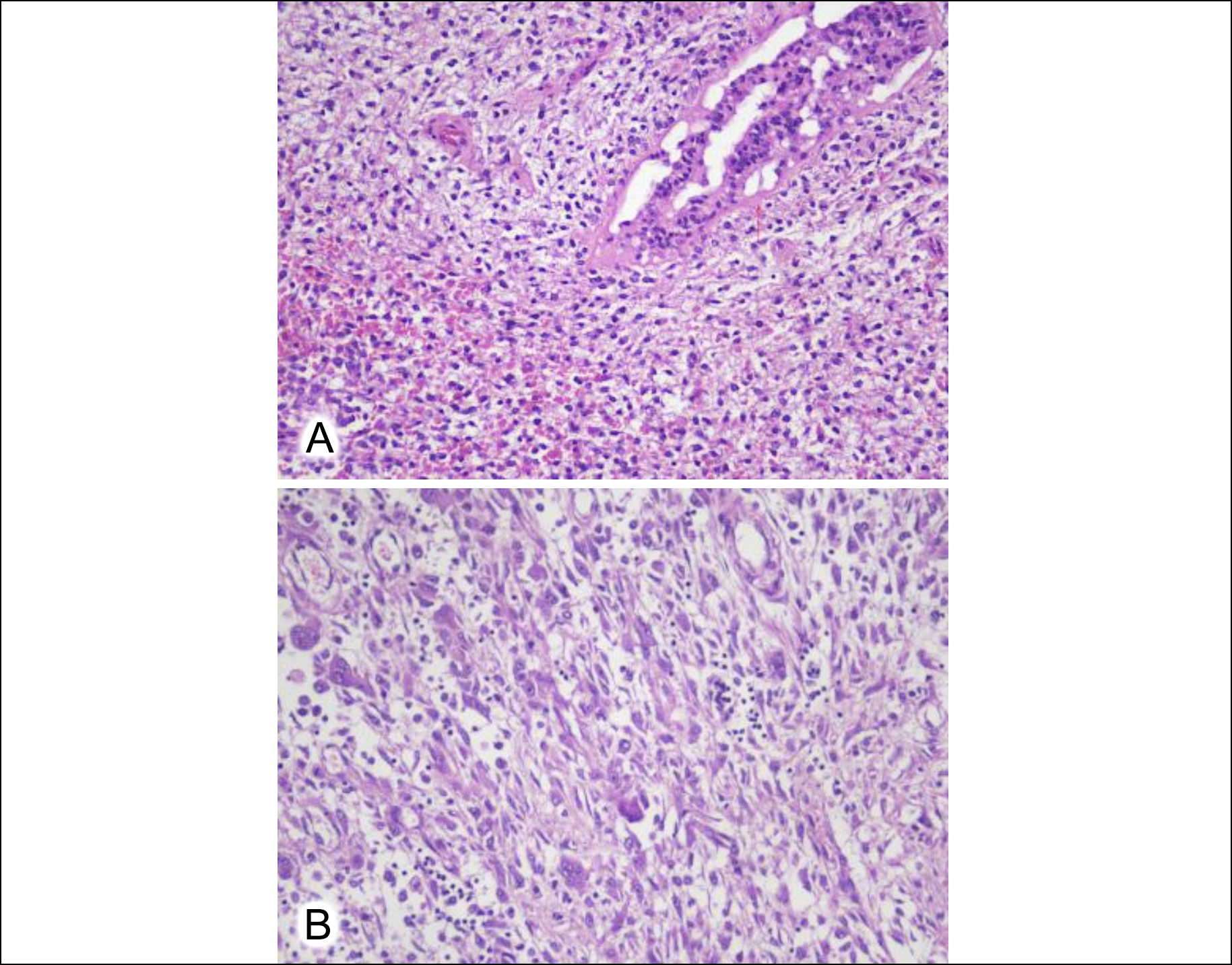 Figure 2: (A) Benign breast duct with cellular stroma (H&E, ×20). (B) Stromal cells admixed with numerous osteoclast-type giant cells (H&E, ×20).
Figure 2: (A) Benign breast duct with cellular stroma (H&E, ×20). (B) Stromal cells admixed with numerous osteoclast-type giant cells (H&E, ×20).
After 6 months, the patient developed a recurrence and simple mastectomy was performed. Grossly, it consisted of a nipple and areola-bearing right breast specimen that measured 21 × 15.5 × 7 cm. The cut surface showed an ulcerated infiltrative lesion in the upper outer quadrant that measured 8 × 7 × 3.5 cm. The cut surface of the lesion was solid-cum-cystic with areas of tan-brown firm nodules. The lesion was 2.6 cm away from the muscle resection margin. Microscopic examination showed a biphasic fibroepithelial lesion with infiltrative margins. There were scant breast ducts lined by epithelial and myoepithelial layers surrounded by expanded cellular stroma (Figure 2A). The stromal cells were spindle to epithelioid having moderate eosinophilic cytoplasm with pleomorphic atypical nuclei. Areas with admixed OGCs and inflammatory cells were present (Figure 2B). Skin ulceration, necrosis and a mitotic count of 14/10 HPFs was noted. Immunostain SATB2 was positive in the stromal cells. The case was diagnosed as MPT with OGCs with a clear resection margin of 2.6 cm. The patient underwent neoadjuvant radiotherapy and was disease-free on a follow-up of 1 year.
DISCUSSION
MPT is an aggressive malignancy, originating from the intralobular and periductal stroma of the breast. PT occurs predominantly in middle-aged females with an age range of 40-50 years; however, its occurrence in Asian women is at a relatively younger age of 25-30 years.8 It usually presents as a unilateral, firm, painless, and mobile mass. Multifocal and bilateral lesions are uncommon.9
OGCs are seen in various types of breast cancers such as fibroadenoma, PT, undifferentiated pleomorphic sarcoma, and invasive carcinoma of the breast.8 OGCs are considered non-neoplastic reactive cells of histiocytic origin. Only a few cases of PT with OGCs are reported in the literature.4,5 The differential diagnoses in cases of breast tumours with giant cells include pleomorphic carcinoma of the breast, metaplastic carcinoma of the breast, carcinoma of the breast with choriocarcinomatous features, invasive ductal carcinoma of the breast with osteoclast-like giant cells, undifferentiated pleomorphic sarcoma, and malignant giant cell tumour of breast/metastatic origin.5
Pleomorphic carcinoma of the breast is characterised by pleomorphic and bizarre tumour cells comprising 50% or more of the tumour population. These cells have marked nuclear pleomorphism with hyperchromasia in contrast to vesicular nuclei in OGCs. Immunostains CKAE1/AE3, CAM5.2, and EMA are positive in the bizarre cells. Metaplastic carcinoma of the breast exhibits neoplastic epithelial differentiation towards squamous or mesenchymal cells, including spindle, chondroid or osteoblastic cells. Metaplastic carcinoma shows both carcinomatous and sarcomatous features. The epithelial component in MPT is benign in contrast to malignant epithelial cells in metaplastic carcinoma. The most difficult to differentiate from MPT is the spindle cell type of metaplastic carcinoma. In such cases, thorough sampling is required to find classic phyllodes areas. Also, broad spectrum and high molecular weight keratins including CKAE1/AE3, CAM5.2, EMA, 34Beta E12, and CK5/6 are positive in 75% of cases of metaplastic carcinoma and negative in MPT. Immunostain p63 is also positive in metaplastic carcinoma.
Invasive carcinoma of the breast with choriocarcinomatous features shows markedly pleomorphic multinucleated choriocarcinomatous cells in an otherwise invasive ductal carcinoma or metaplastic carcinoma. Associated ductal carcinoma in situ may be present. The multinucleated cells are positive for human placental lactogen and beta-HCG in addition to CKAE1/AE3. Invasive ductal carcinoma of the breast with osteoclastic giant cells shows invasive breast carcinoma with OGCs. These giant cells have variable sizes and numbers of nuclei and are positive for immunostain CD68 and negative for CKAE1/AE3, EMA, and S100.
Undifferentiated pleomorphic sarcoma is a type of undifferentiated sarcoma that does not show any lineage-specific differentiation. It shows fascicles and sheets of spindle and bizarre multinucleated giant cells. Epithelial component is absent. Immunostains CKAE1/AE3, ASMA, Desmin, CD34 and S100 are negative and it is a diagnosis of exclusion. Giant cell tumour (GCT) of the soft tissue of the breast is a rare entity. Histologically, GCT of the breast exhibits spindle mononuclear cells admixed with OGCs resembling GCT of bone. Epithelial components and phyllodes-like areas are absent. Diagnosis requires clinical history regarding any previous bone swelling/biopsy and radiological correlation to exclude metastasis.
The treatment recommended for borderline and MPTs is wide excision with a surgical margin of 1 cm or more.10 There is scant data in comparison of breast-conserving surgery with mastectomy in PT. However, where achieving adequate margins is possible with adjuvant radiation therapy, breast-conserving surgery has been as effective as total mastectomy in MPT. The local recurrence rate of MPT ranges from 15% to 40%. Local recurrence typically occurs within two years of the initial excision with a shorter time interval for MPT. In both of the described cases, patients developed local recurrence within 6 months that may be attributed to close margins of excision.
MPT with OGCs is a rare entity with diverse differential diagnoses. The distinction among the entities is important because of significant differences in behaviour, prognosis, treatment, and management of tumours. Thorough sampling and immunohistochemistry help in achieving a definite diagnosis.
PATIENTS' CONSENT:
Written informed consents were obtained from the patients.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
SMA, AR: Conception and drafting of the manuscript.
MBQ: Revision of the manuscript.
NU, NK: Final approval of manuscript.
All the authors have approved the final version of the manuscript to be published.
REFERENCES